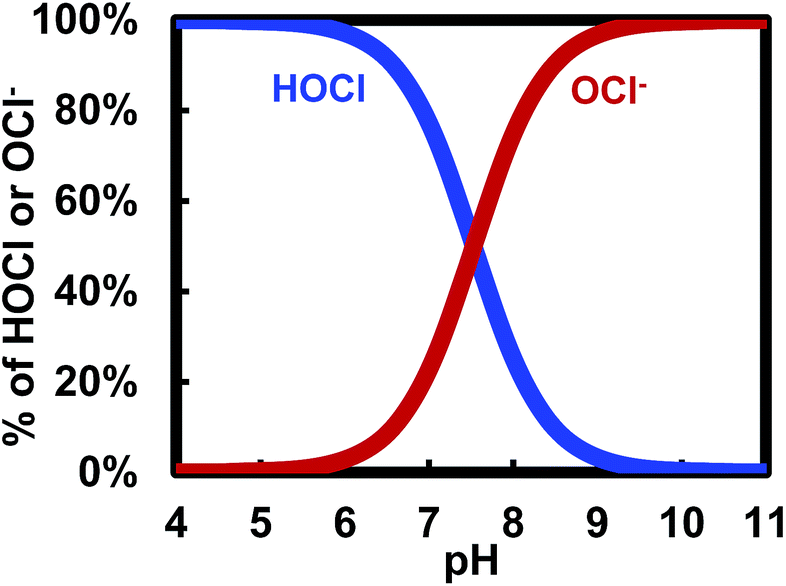
Learn the relationship between chlorine and cyanuric acid, and how cyanuric acid stabilizes the free chlorine in your pool.
Maintaining both pH and total alkalinity in your swimming pool is important for keeping your pool properly sanitized and non-corrosive. Total alkalinity is to pH what cyanuric acid is to free chlorine. Total alkalinity stabilizes pH levels. The ideal pool pH level is 7.4 to 7.6. The ideal total alkalinity level is 80 to 120 ppm.
The pH scale is a measure of acidity or basicity (often called alkalinity) of chemical substances. It is a logarithmic scale that assesses the “potential hydrogen” of a given substance.
The pH scale is valued from 0 to 14 – where lower pH indicates acidity, higher pH indicates alkalinity, and 7 is perfectly neutral (i.e. neither acidic nor alkaline). For reference, fresh water is assumed to have a pH of 7. It is important to understand that because the scale is logarithmic, each whole number represents an order of magnitude difference. For example, a substance with a pH rating of 6 is ten times more acidic than fresh water.
The most important consideration in terms of pool chemistry is that pH dramatically impacts the sanitizing ability of free chlorine. In pool water with pH of 8.0, free chlorine is 20% effective at sanitizing contaminants. In pool water with pH of 7.0, free chlorine is 66% effective at sanitizing contaminants.
Alkalinity is the opposite of acidity. Alkaline materials are chemical compounds that have the potential to raise pH levels. Total alkalinity is measured in parts per million (ppm), just like free chlorine and cyanuric acid. There are numerous substances you may add to your pool that raise the total alkalinity of your pool water.
The most common substance added to your pool water that raises total alkalinity is cyanuric acid (aka chlorine stabilizer). Cyanuric acid stabilizes chlorine from evaporating due to UV sunlight. Learn more about The Relationship Between Pool Chlorine and Cyanuric Acid. However, cyanuric acid is also a buffer. A buffer is anything that causes pH to remain at an elevated level. All alkaline materials are buffers. Cyanuric acid happens to be the most common buffer found in pool water. In effect, cyanuric acid helps stabilize both chlorine and pH. It binds with chlorine to prevent photolysis and it keeps pH elevated.

Learn the relationship between chlorine and cyanuric acid, and how cyanuric acid stabilizes the free chlorine in your pool.
Alkaline materials are an important presence in your pool water because many of the contaminants in pool water are acidic. For example, urine typically has a pH of about 6. Chlorine solutions can also be acidic. For example, trichlor has a pH of about 3. If you use trichlor to sanitize your pool water, you are simultaneously making your pool water more acidic. However, trichlor is a stabilized chorine solution. Therefore, you are adding cyanuric acid – an alkaline material – to buffer your pool water.
The ideal pool pH level is 7.4 to 7.6. When pH is too low, the pool water is acidic. This can cause skin and eye irritation to swimmers. More importantly, acidic pool water can corrode your pool surface and other pool tools you use, such as your pool filter. When pH is too high, free chlorine cannot sanitize as effectively and your pool may be unsafe to use due to contaminants. At a pH of 7.4 to 7.6, your pool water is non-corrosive and comfortable to swim in, and free chlorine sanitizes your pool water with a high degree of efficacy.
The ideal total alkalinity level is 80 to 120 ppm. When total alkalinity is too low, pH is unstable and may oscillate. When total alkalinity is too high, the buffering effect may cause pH to rise and dilute the sanitizing efficacy of free chlorine. At a level of 80 to 120 ppm, total alkalinity buffers pool water to stabilize pH but allows free chlorine to sanitize contaminants effectively.
Use test strips to make sure your pH and total alkalinity are in recommended ranges.

Want to learn more about algaecide? Read on to find out when to add algaecide to your pool maintenance routine and other helpful tips.

In this quick guide, we’ll answer the question “can you over shock a pool” and unveil the factors to consider when shocking a pool.

Maintaining both pH and total alkalinity in your swimming pool is important for keeping your pool properly sanitized and non-corrosive. Total alkalinity is to pH what cyanuric acid is to free chlorine. Total alkalinity stabilizes pH levels. The ideal pool pH level is 7.4 to 7.6. The ideal total alkalinity level is 80 to 120 ppm.

The Association of Pool and Spa Professionals recommends free chlorine levels for both swimming pools and hot tubs be kept between 2.0 and 4.0 ppm. However, the Center for Disease Control recommends free chlorine stay above 1 ppm in pools and 3 ppm in hot tubs.